SARS-CoV-2 IgM/IgG Rapid Test


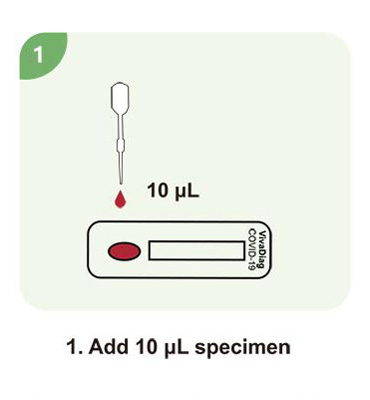
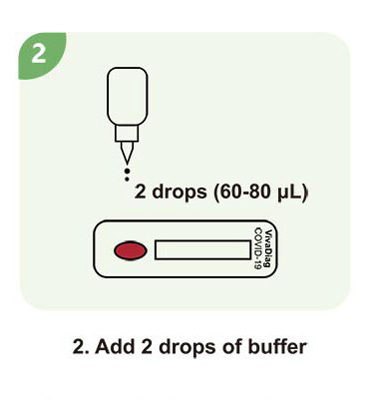
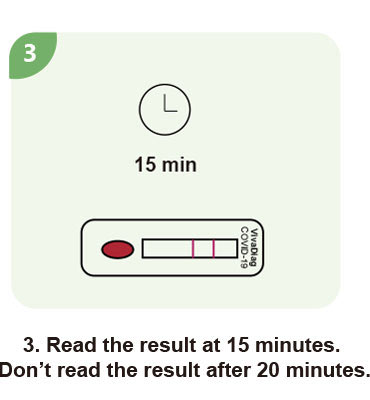
Steps for a single test
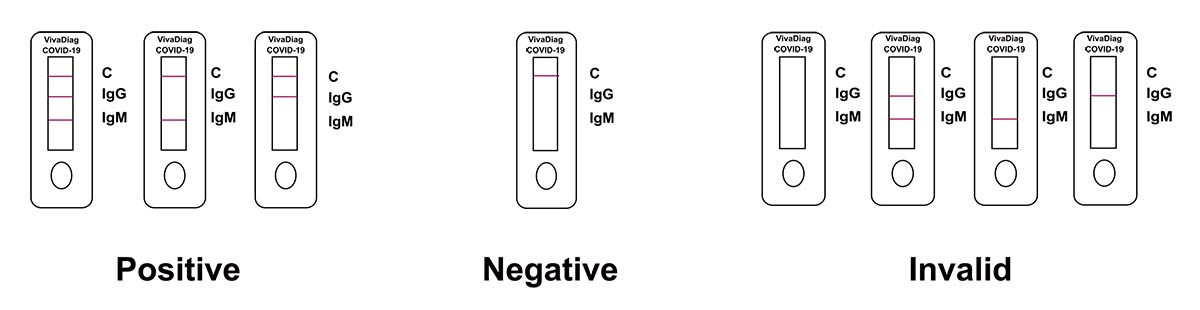
Interpretation of Results
Specification
| Product Name | VivaDiag™ SARS-CoV-2 IgM/IgG Rapid Test (COVID-19 IgM/IgG Rapid Test) |
| Test Principle | Colloidal gold |
| Sample Type | Whole blood (fingertip/venous ), serum or plasma |
| Sample Volume | 10 μL |
| Test Time | 15 min |
| Operation Temperature | 15-30℃ |
| Storage Temperature | 2-30℃ |
| Shelf Life (Unopened) | 24 months |
Not Available for Sales in the US
Independent Evaluation by Authorities and Third Parties
-
COVID-19 Testing Project,
USA
A total of 10 commercially available serological tests are evaluated by a group of researchers and physicians at UCSF, UC Berkeley, Chan Zuckerberg Biohub and Innovative Genomics Institute. -
University Hospitals Leuven, Leadlife B.V., KU Leuven, Belgium
Diagnostic Performance of 7 Rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. - Health Science Agency, Singapore Government
- Therapeutic Goods Administration (TGA), Department of Health, Australian Government
- Foundation for Innovative New Diagnostics (FIND)
**Warning**
NOT FOR AT-HOME TESTING.
The VivaDiag™ SARS-CoV-2 IgM/IgG Rapid Test (COVID-19 IgM/IgG Rapid Test) has ONLY been designed to act as a supplementary test for suspected cases of negative coronavirus nucleic acid detection or in conjunction with nucleic acid detection in the diagnosis of suspected cases. Results from IgM/IgG antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 (COVID-19) infection or to inform infection status.
VivaDiag™ SARS-CoV-2 IgM/IgG Rapid Test (COVID-19 IgM/IgG Rapid Test) IS INTENDED FOR USE BY HEALTHCARE PROFESSIONALS AND CLINICAL LABS ONLY. DO NOT SEPARATE TEST COMPONENTS. IMPROPER USE MAY PRODUCE INVALID AND/OR UNRELIABLE TEST RESULTS.
DO NOT USE IF PACKAGE SEAL HAS BEEN REMOVED OR BROKEN.